
Following dramatic staffing cuts and an overall lack of clarity from federal officials, the future of an important program for recycled plastics remains a question mark. | StanislauV/Shutterstock
Amid sweeping federal staffing cuts, the future of a government program to assess processes to make food packaging from recycled plastics is unclear, sources told Plastics Recycling Update in recent interviews.
The U.S. Food and Drug Administration considers uses of recycled polymers on a case-by-case basis and issues informal advice on whether the recycling process is expected to produce plastic of suitable purity for food-contact applications, according to the agency website. When the FDA issues a favorable opinion on the suitability of the process, it’s known as a letter of no objection or no objection letter, known as an LNO or NOL.
In 2024, 53 LNOs were issued, higher by 20, or 61%, from the previous year and an all-time high since the first in 1990. In the nearly 35 years since the program began, fewer than 400 LNOs have been issued, with nearly one-third of them occurring in 2022-2024. But at press time the public FDA database had not been updated since the end of 2024, an unusual gap.
As seen in an interactive analysis prepared by Plastics Recycling Update, certain trends have emerged, such as a broadening slate of polymers submitted as well as countries of origin. Some of those trends are likely a result of increased domestic demand, with most consumer brands setting voluntary PCR targets and several states approving extended producer responsibility programs for packaging, with more on the way.
In addition to EPR and voluntary brand-owner targets, the increase in LNO submissions reflect markets that may be opening up, said Cynthia Lieberman, partner at Keller & Heckman law firm. For example, in 2021 India reversed its ban on using recycled plastics in food-contact applications, potentially driving technologies to be developed in those regions.
Explore the Data
- View an interactive analysis of LNO data prepared by Plastics Recycling Update.
- View LNOs received for Total Corbion, Fresh Pak and KW Plastics.
While important, the process is entirely voluntary, both in submitting applications and the government’s obligation to review them. In interviews with Plastics Recycling Update, several industry stakeholders provided their perspectives on the current process as well as what may lie ahead.
What an LNO is – and what it isn’t
Although the terms may be used interchangeably among industry stakeholders, an LNO does not constitute FDA approval or certification but rather is a first step on the road to creating high-quality recycled content that is safe for food contact.
“The way the law is structured, it’s on the companies to ensure compliance of the recycled plastics they’re using in their packaging, in the same way that they would ensure compliance of the virgin material, and that’s often just passed down the supply chain to either the virgin producers or the recyclers to be doing appropriate testing and quality testing and compliance testing to ensure that they are in fact suitable for use of contact with food,” Lieberman said.
Although legally speaking the process is voluntary, “from a market standpoint, it’s a very important piece of paper to have. Customers like to see that the FDA has reviewed your recycling process,” said Martha Marrapese, partner at Wiley law firm. “It’s an extra level of assurance for customers, and it makes it easier sometimes to get customers to use your products.”
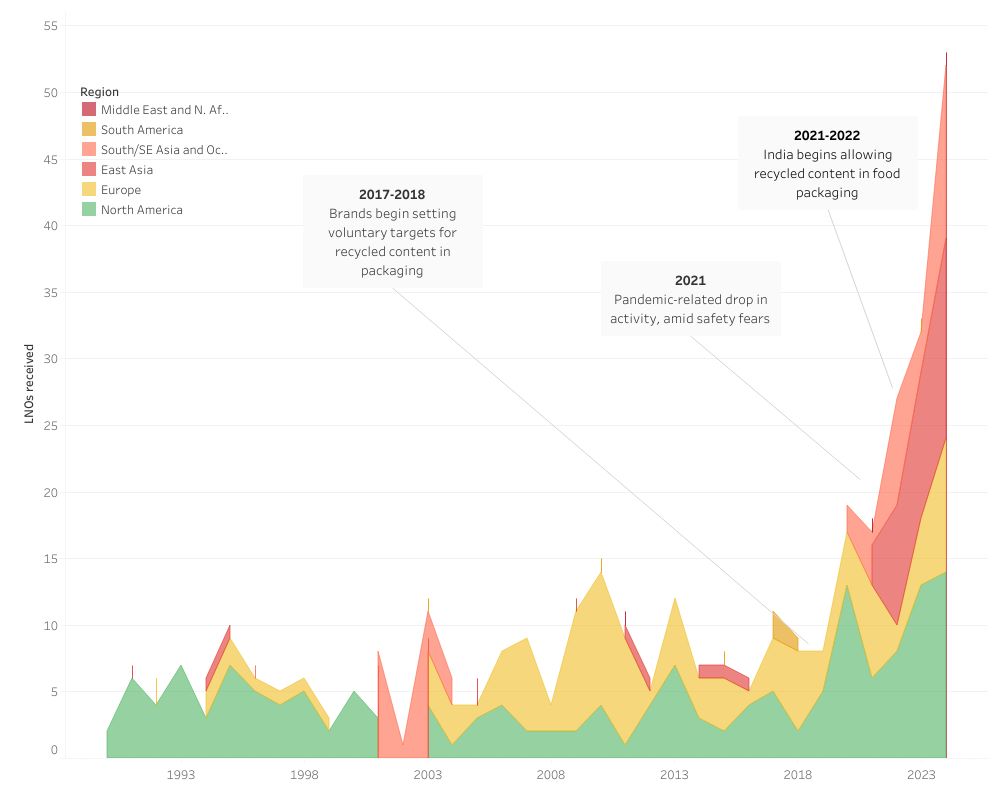
Find the interactive version here. Credit: Antoinette Smith, Plastics Recycling Update
Crystal Bayliss, director of strategy and engagement at the U.S. Plastics Pact, agreed: “It’s an important tool, but for a lot of companies, it’s also only a starting point. It’s kind of a snapshot in time of what the processes are.”
Food companies and even personal care brands would likely require an LNO to begin considering PCR for their packaging, Bayliss added, “but then they’ll also look to do additional testing, likely on an ongoing basis, of the lots coming in, to just continue to confirm that everything is safe for the products that are going into it.”
In addition, potential resin buyers must examine LNOs carefully, as they are issued for a specific set of food types and conditions. Shannon Gordon, chief operating officer of procurement platform Circular.co, said her clients may be looking for a recycled material for a particular use, but the available resins are not approved for that use. “So it’s not just a yes, no, are you FDA certified?” she said.
Current administration priorities
The new administration has terminated thousands of federal workers in recent months, including about 20% of the workforce at the FDA, an agency within the Department of Health and Human Services. While many of the terminations are being challenged in court and some workers have been rehired, current and future staffing levels are anything but clear, especially when considered in tandem with inconsistent and unpredictable messaging within the administration.
HHS did not respond to a request for comment.
The potential for reduced staffing to review LNOs could be problematic, Lieberman said, a sentiment echoed in a recent podcast from the firm.
“If there are non-statutory programs the agency is undertaking, it’s their intention to stop the agency from doing that work even if it’s for the public good,” partner George Misko said on the podcast. So for a task with no legal mandate, “if someone sees an opportunity to reassign man hours, they may do that.”
In an interview, Lieberman said, “I think it’s only sort of logical to expect that something that is a voluntary program would be higher up on the programs on the chopping block if they were to see significant resource constraints in terms of their personnel.”
She added, “I’m not suggesting in any way that we expect them to, you know, get rid of the program. But I think it is reasonable to expect that you could start seeing delays in the timelines for which those LNOs are evaluated and posted. So that may lead to fewer being posted, just simply because the agency is taking longer to do them because they don’t have as many people on staff reviewing them.”
The FDA database normally is updated about every month or so, Lieberman said. Despite the recent gap, Wiley’s Marrapese said she knew of several LNOs issued since inauguration day in January.
“There are unknowns regarding the impact of the recent changes at FDA and whether additional changes may happen,” she said. “But the plastic recycling program continues to operate, and I’m not aware of any plans to alter the status of that program. So I’d encourage stakeholders to continue their recycling submissions and continue to rely on the LNO process.”
HHS Sec. Robert F. Kennedy Jr. “is very focused, maybe more so than previous cabinet secretaries, on the food side,” Lieberman said, adding that although the Human Foods Program likely had experienced staffing cuts, she didn’t think they were as stark as those for, say, tobacco-related programs.
Bayliss at the U.S. Plastics Pact added that at the April Chemicals of Concern Policy Summit, Kennedy discussed reviewing FDA processes — specifically the Generally Recognized As Safe process — to ensure it reflected industry innovations. “And so I do think to a certain extent, any process within the FDA is up for reconsideration, just to make sure, and I think that that’s important, because you really can innovate faster as a company than the government processes.”
And the FDA’s priorities seem to be more in the area of direct food additives, children’s health, and drugs and vaccines, Marrapese said. “So they have a long list to get to before they even hopefully think about this recycling program, and it’s working well, so hopefully they will recognize that.”
What’s the alternative?
Although so far the LNO process seems to be continuing, because of its status as a voluntary program, “there’s nothing that would prevent a company from making a compliance determination of the recycled material for use in their intended packaging applications,” Lieberman said. “FDA doesn’t necessarily have any sort of stronghold on this. I think it was a convenience that they had started for the industry and dating back a couple of decades now.”
Bayliss added that if the LNO process were discontinued, “I think the industry would be reeling for a while, because there’s not just a standing thing that you could shift to.” But because an LNO is only the first part of a multi-step process, “in theory, the industry can try to standardize — which I think would actually be helpful overall — what type of testing is required.”
But that wouldn’t happen overnight, she said. “And I do think it would be a pretty significant disservice to all of the reclaimers, because they really need that LNO to have a starting point for potential qualification.”
Marrapese said that regardless of the future of the LNO program, existing private testing options remain, including surrogate contaminant testing. “And so companies can always continue to do the necessary testing themselves and work with experts in the field to make determinations that their packaging materials are suitably pure for use in contact with food.”
That said, the current uncertainty raises the question of the extent to which the government should allow companies to review and qualify their own materials — especially chemical additives — without government review, she added. “But right now that is still allowed.”
However, Bayliss said, “whether it’s virgin plastic or PCR, brand owners don’t want to go in it alone, because the legal risk is just so high, and so it’s important, I think, for the government to continue to offer the LNO and to continue to update the process and the requirements based off of research as it’s coming out, to make sure that we’re keeping up with changes in the industry.
“And if we’re going to advance the use of PCR, brand owners really need to know that they’re covered from a legal standpoint, because they’re not having to tackle these complex subjects on their own.”
In April, the U.S. Plastics Pact released a report on scaling up use of recycled content in packaging. As part of its roadmap toward this goal, the report recommended that the FDA:
- Receive funding to expedite LNO evaluations, setting a mandatory 180-day review period and increasing staff capacity to process applications efficiently.
- Update guidance on recycled plastics in food packaging more frequently to reflect rapidly evolving research on chemicals of concern, such as phthalates, bisphenols and PFAS. The most recent update was in 2021.
In the end, amid so much uncertainty, “I think we’re all sort of taking a bit of a wait-and-see approach,” Lieberman said.

