Researchers at the Department of Energy’s Pacific Northwest National Laboratory are recovering manganese, magnesium, dysprosium and neodymium from e-scrap using a salt water-based solution. | Mike Perkins/Pacific Northwest National Laboratory
Researchers at the Department of Energy’s Pacific Northwest National Laboratory found a way to recover some critical metals from e-scrap using a mixed-salt water-based solution.
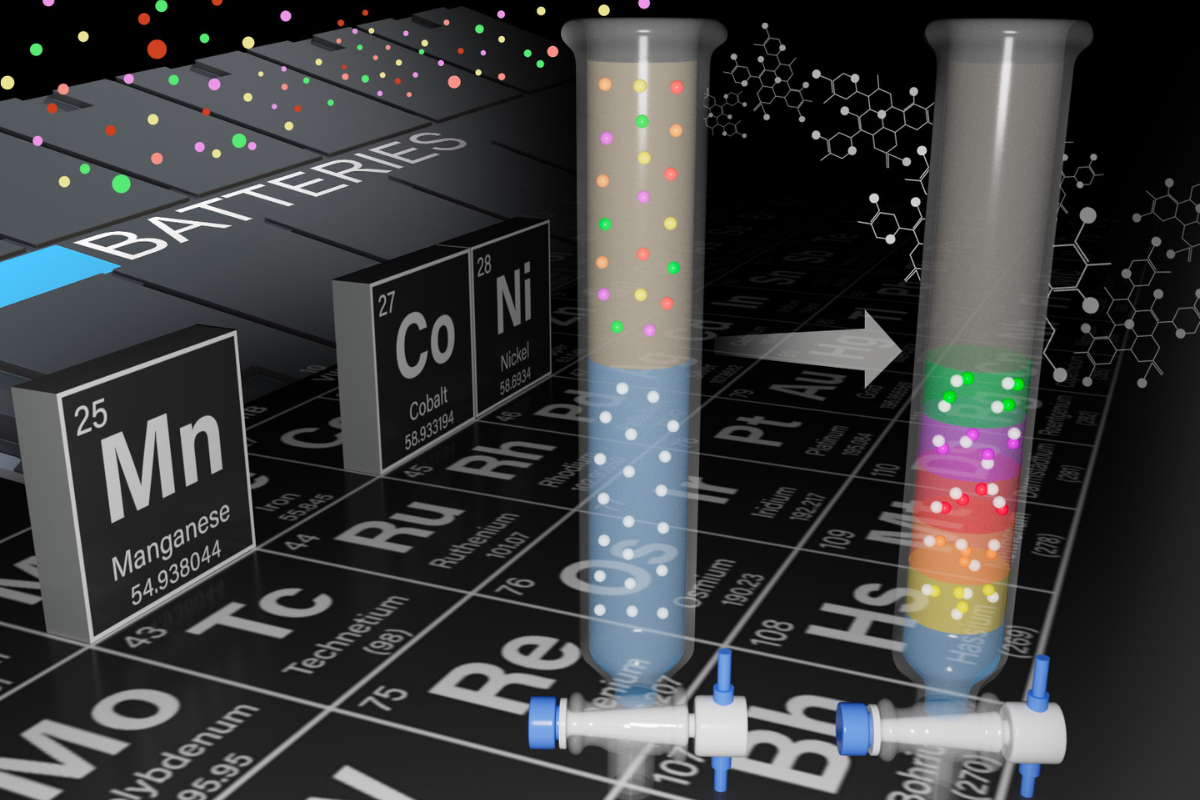
Materials separation scientist Qingpu Wang led a team to develop this method to selectively recover manganese, magnesium, dysprosium and neodymium by dissolving the e-scrap containing them in continuously flowing reaction chambers, a press release noted.
The method relies on the behavior of metals when placed in a reaction chamber in which two different liquids are flowing together. Dissolved metals will form solids at different rates, which the researchers used to separate and purify them.
“Our goal is to develop an environmentally friendly and scalable separation process to recover valuable minerals from e-waste,” Wang said. “Here we showed that we can spatially separate and recover nearly pure rare earth elements without complex, expensive reagents or time-consuming processes.”
The first success occurred in February, when the researchers successfully separated neodymium and dysprosium from a mixed liquid. The separation process took four hours using their method, while conventional separation methods typically take around 30 hours.
Wang said the next goal is to modify the design of the reactor to recover a larger amount of product.
Building off that research and using a complementary technique, Wang and materials scientist Elias Nakouzi recovered nearly pure manganese from a solution that mimicked dissolved lithium-ion battery waste.
They used a gel-based system that relied on the different transport and reactivity rates of the metals in the sample.
“The beauty in this process is its simplicity,” Nakouzi said. “Rather than relying on high-cost or specialty materials, we pared things back to thinking about the basics of ion behavior. And that’s where we found inspiration.”

